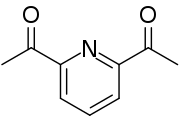
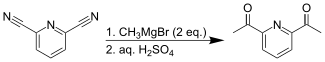2,6-二乙醯基吡啶
外觀
| 2,6-Diacetylpyridine | |
|---|---|
| |

| |
| IUPAC名 1,1′-(Pyridine-2,6-diyl)di(ethan-1-one) | |
| 別名 | 1,1′-(Pyridine-2,6-diyl)diethanone 1-(6-Acetylpyridin-2-yl)ethanone DAP 2,6-Bisacetylpyridine |
| 識別 | |
| CAS號 | 1129-30-2 |
| PubChem | 70790 |
| ChemSpider | 63955 |
| SMILES |
|
| 性質 | |
| 化學式 | C9H9NO2 |
| 摩爾質量 | 163.17 g·mol−1 |
| 外觀 | 白色晶體 |
| 密度 | 1.119 g/cm3 |
| 熔點 | 81 °C |
| 沸點 | 126 °C 110至130 °C(230至266 °F;383至403 K)(升華) |
| 危險性 | |
GHS危險性符號
| |
| GHS提示詞 | Warning |
| H-術語 | H315, H319, H335 |
| P-術語 | P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405 |
| 相關物質 | |
| 相關化合物 | 2-acetylpyridine |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
2,6-二乙醯基吡啶是一種有機化合物,化學式為C9H9NO2。它可由以下兩種方法製備:[1][2]
它和鹽酸羥胺在鹼存在下反應,可以得到相應的肟(羥亞胺);[3]它和鹽酸苯肼反應,可以得到相應的苯腙。[4]它可以被硼氫化鈉還原為α,α'-二甲基-2,6-吡啶二甲醇。[5]
參考文獻
[編輯]- ^ Yoshiro Ogata; Masaru Tsuchida; Akihiko Muramoto. Controlled Synthesis of 2-Acetyl-6-carbethioxypyridine and 2-6-Diacetylpyridine from 2,6-Dimethylpyridine. Synth. Commun. 2006, 35 (17): 2317–2324. S2CID 93168188. doi:10.1080/00397910500186995.
- ^ Schmidt, R.; Welch, M.B.; Palackal, S.J.; Alt, H.G. Hydrogenized iron(II) complexes as highly active ethene polymerization catalysts. Journal of Molecular Catalysis A: Chemical. 2001, 179 (1–2): 155–173. doi:10.1016/S1381-1169(01)00333-8.
- ^ Christer B. Aakeröy, Abhijeet S. Sinha. Synthesis of ketoximes via a solvent-assisted and robust mechanochemical pathway. RSC Advances. 2013, 3 (22): 8168 [2022-03-15]. ISSN 2046-2069. doi:10.1039/c3ra40585k (英語).
- ^ L. H. Abdel-Rahman, A. M. Abu-Dief, F. M. Atlam, A. A. H. Abdel-Mawgoud, A. A. Alothman, A. M. Alsalme, A. Nafady. Chemical, physical, and biological properties of Pd(II), V(IV)O, and Ag(I) complexes of N 3 tridentate pyridine-based Schiff base ligand. Journal of Coordination Chemistry. 2020-12-01, 73 (23): 3150–3173 [2022-03-15]. ISSN 0095-8972. doi:10.1080/00958972.2020.1842378 (英語).
- ^ Jun'ichi Uenishi, Sachiko Aburatani, Taro Takami. Stereocomplexity and Stereoselective Synthesis of Triamine Molecules Bearing Four Chiral Carbon Centers: Stereodifferentiated Preparation of All 10 Stereoisomers of 2,6-Bis[1-(1-phenylethylamino)ethyl]pyridines. The Journal of Organic Chemistry. 2007-01-01, 72 (1): 132–138 [2022-03-15]. ISSN 0022-3263. doi:10.1021/jo061729e. (原始內容存檔於2022-03-15) (英語).