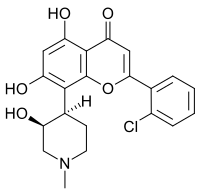
阿伏西地
外观
| 阿伏西地 | |
|---|---|
| |
| |
| IUPAC名 2′-Chloro-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]flavone | |
| 系统名 2-(2-Chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]-4H-1-benzopyran-4-one | |
| 别名 | Flavopiridol, HMR 1275, L-868275 |
| 识别 | |
| CAS号 | 146426-40-6 |
| PubChem | 5287969 |
| ChemSpider | 4450222 |
| SMILES |
|
| ChEBI | 47344 |
| DrugBank | DB03496 |
| KEGG | D09868 |
| MeSH | Flavopiridol |
| IUPHAR配体 | 5680 |
| 性质 | |
| 化学式 | C21H20ClNO5 |
| 摩尔质量 | 401.8402 g·mol⁻¹ |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
阿伏西地(INN:alvocidib;开发代号:HMR 1275、L-868275),也叫夫拉平度(flavopiridol),是一种黄酮类生物碱CDK9激酶抑制剂,由Tolero制药公司进行临床开发,用于治疗急性骨髓性白血病。还研究了将它用于治疗关节炎[1]和粥样斑块形成。[2]阿伏西地的靶点是正转录延伸因子(P-TEFb)。[3][4]用阿伏西地处理细胞会抑制P-TEFb并导致mRNA产生的损失。[5][6]
该化合物是罗希吐碱的合成类似物。罗希吐碱是一种天然产物,最初从山楝中提取,后来从红果㭴木中提取。[7][8]
孤儿药
[编辑]美国食品药品监督管理局已授予阿伏西地孤儿药资格,用于治疗急性髓系白血病患者。[9]
参考资料
[编辑]- ^ Sekine C, Sugihara T, Miyake S, Hirai H, Yoshida M, Miyasaka N, Kohsaka H. Successful treatment of animal models of rheumatoid arthritis with small-molecule cyclin-dependent kinase inhibitors. J. Immunol. 2008, 180 (3): 1954–61. PMID 18209094. doi:10.4049/jimmunol.180.3.1954
 .
.
- ^ Ruef J, Meshel AS, Hu Z, Horaist C, Ballinger CA, Thompson LJ, Subbarao VD, Dumont JA, Patterson C. Flavopiridol inhibits smooth muscle cell proliferation in vitro and neointimal formation In vivo after carotid injury in the rat. Circulation. 1999, 100 (6): 659–65. PMID 10441105. doi:10.1161/01.cir.100.6.659
 .
.
- ^ Chao SH, Fujinaga K, Marion JE, Taube R, Sausville EA, Senderowicz AM, Peterlin BM, Price DH. Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J. Biol. Chem. 2000, 275 (37): 28345–8. PMID 10906320. doi:10.1074/jbc.C000446200
 .
.
- ^ Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J. Biol. Chem. 2001, 276 (34): 31793–9. PMID 11431468. doi:10.1074/jbc.M102306200
 .
.
- ^ Cheng B, Li T, Rahl PB, Adamson TE, Loudas NB, Guo J, Varzavand K, Cooper JJ, Hu X, Gnatt A, Young RA, Price DH. Functional association of Gdown1 with RNA polymerase II poised on human genes. Mol. Cell. 2012, 45 (1): 38–50. PMC 3259526
 . PMID 22244331. doi:10.1016/j.molcel.2011.10.022.
. PMID 22244331. doi:10.1016/j.molcel.2011.10.022.
- ^ Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA. c-Myc regulates transcriptional pause release. Cell. 2010, 141 (3): 432–45. PMC 2864022
 . PMID 20434984. doi:10.1016/j.cell.2010.03.030.
. PMID 20434984. doi:10.1016/j.cell.2010.03.030.
- ^ Harmon, AD; Weiss, U; Silverton, JV. The structure of rohitukine, the main alkaloid of Amoora rohituka (syn.Aphanamixis polystachya) (Meliaceae). Tetrahedron Lett. 1979, 20 (1): 721–724. doi:10.1016/S0040-4039(01)93556-7.
- ^ Lakdawala, AD; Shirole, MV; Mandrekar, SS; Dohadwalla, AN. Immunopharmacological potential of rohitukine: a novel compound isolated from the plant Dysoxylum binectariferum. Asia Pac J Pharmcol. 1988, 3 (1): 91–98.
- ^ FDA grants orphan drug status to Alvocidib for AML. [2024-05-03]. (原始内容存档于2015-09-24).
