乙酰膽鹼酯酶
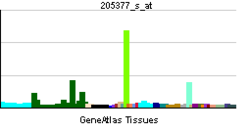
外觀
| 乙酰膽鹼酯酶 | |||||||
|---|---|---|---|---|---|---|---|

| |||||||
| |||||||
| 識別碼 | |||||||
| EC編號 | 3.1.1.7 | ||||||
| CAS號 | 9000-81-1 | ||||||
| 數據庫 | |||||||
| IntEnz | IntEnz瀏覽 | ||||||
| BRENDA | BRENDA入口 | ||||||
| ExPASy | NiceZyme瀏覽 | ||||||
| KEGG | KEGG入口 | ||||||
| MetaCyc | 代謝路徑 | ||||||
| PRIAM | 概述 | ||||||
| PDB | RCSB PDB PDBj PDBe PDBsum | ||||||
| 基因本體 | AmiGO / EGO | ||||||
| |||||||
乙酰膽鹼酯酶(acetylcholinesterase,AChE,AChase),EC 3.1.1.7,是催化神經遞質乙酰膽鹼降解(通過其水解活性)成膽鹼和乙酸的酶;屬於一種水解酶或絲氨酸蛋白酶或羧酸酯酶家族[1]。
此酶主要存在於膽鹼能神經元、神經肌肉接頭處、肌肉組織等,具終止乙酰膽鹼對突觸後膜興奮作用,恢復突觸後膜極化,可終止突觸傳遞。
乙酰膽鹼酯酶具有極高的水解活性——一分子的乙酰膽鹼酯酶每秒鐘可以水解25000分子的乙酰膽鹼。經乙酰膽鹼酯酶作用而產生的膽鹼被重新利用——通過再攝取被轉運進入神經末梢,在那裏被重新利用以合成新的乙酰膽鹼分子[2]。
參考文獻
[編輯]- ^ 乙酰胆碱酯酶. 術語在線. 全國科學技術名詞審定委員會. (簡體中文)
- ^ Dale Purves, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White. Neuroscience. 4th ed.. Sinauer Associates. 2008: 121–2. ISBN 978-0-87893-697-7.
延伸閱讀
[編輯]- Silman I, Futerman AH. Modes of attachment of acetylcholinesterase to the surface membrane. Eur. J. Biochem. 1988, 170 (1-2): 11–22. PMID 3319614. doi:10.1111/j.1432-1033.1987.tb13662.x.
- Soreq H, Seidman S. Acetylcholinesterase--new roles for an old actor. Nat. Rev. Neurosci. 2001, 2 (4): 294–302. PMID 11283752. doi:10.1038/35067589.
- Shen T, Tai K, Henchman RH, McCammon JA. Molecular dynamics of acetylcholinesterase.. Acc. Chem. Res. 2003, 35 (6): 332–40. PMID 12069617. doi:10.1021/ar010025i.
- Pakaski M, Kasa P. Role of acetylcholinesterase inhibitors in the metabolism of amyloid precursor protein. Current drug targets. CNS and neurological disorders. 2003, 2 (3): 163–71. PMID 12769797. doi:10.2174/1568007033482869.
- Meshorer E, Soreq H. Virtues and woes of AChE alternative splicing in stress-related neuropathologies. Trends Neurosci. 2006, 29 (4): 216–24. PMID 16516310. doi:10.1016/j.tins.2006.02.005.
- Ehrlich G, Viegas-Pequignot E, Ginzberg D et al. Mapping the human acetylcholinesterase gene to chromosome 7q22 by fluorescent in situ hybridization coupled with selective PCR amplification from a somatic hybrid cell panel and chromosome-sorted DNA libraries. Genomics. 1992, 13 (4): 1192–7. PMID 1380483. doi:10.1016/0888-7543(92)90037-S.
- Spring FA, Gardner B, Anstee DJ. Evidence that the antigens of the Yt blood group system are located on human erythrocyte acetylcholinesterase. Blood. 1992, 80 (8): 2136–41. PMID 1391965.
- Shafferman A, Kronman C, Flashner Y; et al. Mutagenesis of human acetylcholinesterase. Identification of residues involved in catalytic activity and in polypeptide folding. J. Biol. Chem. 1992, 267 (25): 17640–8. PMID 1517212.
- Getman DK, Eubanks JH, Camp S et al. The human gene encoding acetylcholinesterase is located on the long arm of chromosome 7.. Am. J. Hum. Genet. 1992, 51 (1): 170–7. PMC 1682883
 . PMID 1609795.
. PMID 1609795. - Li Y, Camp S, Rachinsky TL et al. Gene structure of mammalian acetylcholinesterase. Alternative exons dictate tissue-specific expression.. J. Biol. Chem. 1992, 266 (34): 23083–90. PMID 1744105.
- Velan B, Grosfeld H, Kronman C et al. The effect of elimination of intersubunit disulfide bonds on the activity, assembly, and secretion of recombinant human acetylcholinesterase. Expression of acetylcholinesterase Cys-580----Ala mutant. J. Biol. Chem. 1992, 266 (35): 23977–84. PMID 1748670.
- Soreq H, Ben-Aziz R, Prody CA; et al. Molecular cloning and construction of the coding region for human acetylcholinesterase reveals a G + C-rich attenuating structure. Proc. Natl. Acad. Sci. U.S.A. 1991, 87 (24): 9688–92. PMC 55238
 . PMID 2263619. doi:10.1073/pnas.87.24.9688.
. PMID 2263619. doi:10.1073/pnas.87.24.9688. - Chhajlani V, Derr D, Earles B et al. Purification and partial amino acid sequence analysis of human erythrocyte acetylcholinesterase. FEBS Lett. 1989, 247 (2): 279–82. PMID 2714437. doi:10.1016/0014-5793(89)81352-3.
- Lapidot-Lifson Y, Prody CA, Ginzberg D et al. Coamplification of human acetylcholinesterase and butyrylcholinesterase genes in blood cells: correlation with various leukemias and abnormal megakaryocytopoiesis.. Proc. Natl. Acad. Sci. U.S.A. 1989, 86 (12): 4715–9. PMC 287342
 . PMID 2734315. doi:10.1073/pnas.86.12.4715.
. PMID 2734315. doi:10.1073/pnas.86.12.4715. - Bazelyansky M, Robey E, Kirsch JF. Fractional diffusion-limited component of reactions catalyzed by acetylcholinesterase. Biochemistry. 1986, 25 (1): 125–30. PMID 3954986. doi:10.1021/bi00349a019.
- Gaston SM, Marchase RB, Jakoi ER. Brain ligatin: a membrane lectin that binds acetylcholinesterase. J. Cell. Biochem. 1982, 18 (4): 447–59. PMID 7085778. doi:10.1002/jcb.1982.240180406.
- Ordentlich A, Barak D, Kronman C et al. Contribution of aromatic moieties of tyrosine 133 and of the anionic subsite tryptophan 86 to catalytic efficiency and allosteric modulation of acetylcholinesterase. J. Biol. Chem. 1995, 270 (5): 2082–91. PMID 7836436. doi:10.1074/jbc.270.5.2082.
- Maruyama K, Sugano S. Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides. Gene. 1994, 138 (1-2): 171–4. PMID 8125298. doi:10.1016/0378-1119(94)90802-8.
- Ben Aziz-Aloya R, Sternfeld M, Soreq H. Promoter elements and alternative splicing in the human ACHE gene.. Prog. Brain Res. 1994, 98: 147–53. PMID 8248502.
- Massoulie J, Pezzementi L, Bon S, Krejci E, Valette F. Molecular and Cellular Biology of Cholinesterases. Prog. Brain Res. 1993, 93 (1): 31–91. PMID 8321908.
外部連結
[編輯]- ATSDR Case Studies in Environmental Medicine: Cholinesterase Inhibitors, Including Insecticides and Chemical Warfare Nerve Agents(頁面存檔備份,存於互聯網檔案館) U.S. Department of Health and Human Services
- Proteopedia Acetylcholinesterase
- Proteopedia AChE_inhibitors_and_substrates
- Proteopedia AChE_inhibitors_and_substrates_(Part_II)
- Proteopedia AChE bivalent inhibitors AChE_bivalent_inhibitors AChE bivalent inhibitors