伏立诺他
外观
 | |
| 临床资料 | |
|---|---|
| 读音 | /vɒˈrɪnoʊstæt/ vorr-IN-oh-stat |
| 商品名 | Zolinza |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607050 |
| 核准状况 |
|
| 给药途径 | Oral (capsules) |
| ATC码 | |
| 法律规范状态 | |
| 法律规范 |
|
| 药物动力学数据 | |
| 生物利用度 | 1.8–11%[1] |
| 血浆蛋白结合率 | ~71% |
| 药物代谢 | Hepatic glucuronidation and β-oxidation CYP system not involved |
| 代谢产物 | vorinostat O-glucuronide, 4-anilino-4-oxobutanoic acid (both inactive)[2] |
| 生物半衰期 | ~2 hours (vorinostat and O-glucuronide), 11 hours (4-anilino-4-oxobutanoic acid) |
| 排泄途径 | Renal (negligible) |
| 识别信息 | |
| |
| CAS号 | 149647-78-9 |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.207.822 |
| 化学信息 | |
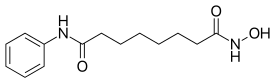
| 化学式 | C14H20N2O3 |
| 摩尔质量 | 264.33 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
伏立诺他(rINN:Vorinostat,商品名:Zolinza)是一种含氮有机化合物[3],分子式C14H20N2O3,是一种组蛋白脱乙酰酶抑制剂。
参考文献
[编辑]- ^ Withdrawal Assessment Report for Vorinostat MSD 100 mg Hard Capsules (vorinostat) (PDF). European Medicines Agency: 9. 23 October 2008 [1 September 2016]. (原始内容 (PDF)存档于15 September 2016).
- ^ Zolinza (vorinostat) Capsules. Full Prescribing Information (PDF). Merck & Co., Inc., Whitehouse Station, NJ 08889, USA. [1 September 2016].
- ^ International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 56 (PDF). WHO Drug Information. 2006, 20 (3): 232 [1 September 2016]. (原始内容 (PDF)存档于July 5, 2011).
外部链接
[编辑]- Vorinostat bound to proteins in the PDB
| ||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
