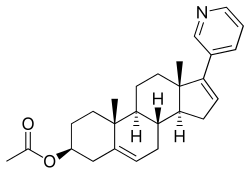
醋酸阿比特龍
外觀
 | |
 | |
| 臨床資料 | |
|---|---|
| 讀音 | a" bir a' ter one |
| 商品名 | Zytiga, Yonsa, others |
| 其他名稱 | CB-7630; JNJ-212082; 17-(3-Pyridinyl)androsta-5,16-dien-3β-ol acetate, abiraterone (BAN UK), abiraterone acetate (JAN JP), abiraterone acetate (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611046 |
| 核准狀況 | |
| 懷孕分級 | |
| 給藥途徑 | 口服給藥[2][3] |
| 藥物類別 | 化學療法 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 生物利用度 | 不明,但空腹時可能最多50%[7] |
| 血漿蛋白結合率 | 阿比特龍:~99.8%(與白蛋白和α1 酸性糖蛋白)[7][2][8] |
| 藥物代謝 | 酯酶、CYP3A4、SULT2A1[8] |
| 代謝產物 | 阿比特龍、其它[2][7] |
| 生物半衰期 | Abiraterone: 12–24小時[2][7][3] |
| 排泄途徑 | 糞便:88%[2][8] 尿:5%[2][8][3] |
| 識別資訊 | |
| |
| CAS號 | 154229-18-2 (154229-19-3(阿比特龍)) |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.063 |
| 化學資訊 | |
| 化學式 | C26H33NO2 |
| 摩爾質量 | 391.56 g·mol−1 |
| 3D模型(JSmol) | |
| 熔點 | 144至145 °C(291至293 °F) [9] |
| |
| |
醋酸阿比特龍(英語:abiraterone acetate)是治療前列腺癌的口服藥物[10]。會與皮質類固醇併用於治療轉移性去勢療法無效型的前列腺癌(metastatic castration-resistant prostate cancer,mCRPC)和轉移性去勢療法敏感型的高風險前列腺癌(metastatic castration-sensitive prostate cancer,mCSPC)[11]。 應使用於睾丸切除(去勢療法)後,或需與促性腺素釋素調節劑併用[11]。
常見副作用包括疲勞、嘔吐、頭痛、關節痛、高血壓、水腫、低鉀血症、高血糖、潮熱、腹瀉和咳嗽[10] [11]。其他嚴重副作用包括可能肝衰竭和腎上腺皮質功能不全[11]。對伴侶可能懷孕的男性,應採取避孕措施[11]。醋酸阿比特龍服用後,在體內轉化為阿比特龍[11]。它是一種雄性激素抑制劑,具體來說是CYP17A1抑制劑之一,作用後會減少睾酮的合成[10]。因此,可以避免這些激素刺激前列腺癌細胞的增生[10]。
醋酸阿比特龍的文獻在 1995 年出現,於 2011 年在美國和歐洲取得醫療使用許可[12] [11]。名列世界衛生組織基本藥物標準清單[13]。此藥在世界各地都有銷售[14]。
參考文獻
[編輯]- ^ Abiraterone Use During Pregnancy. Drugs.com. 13 March 2020 [8 June 2020]. (原始內容存檔於25 November 2020).
- ^ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Zytiga- abiraterone acetate tablet, film coated. DailyMed. 13 June 2019 [15 November 2019]. (原始內容存檔於13 November 2014).
- ^ 3.0 3.1 3.2 3.3 Yonsa- abiraterone acetate tablet. DailyMed. 5 June 2018 [15 November 2019]. (原始內容存檔於13 August 2020).
- ^ 4.0 4.1 4.2 Zytiga abiraterone acetate product information (PDF). TGA eBusiness Services. Janssen-Cilag Pty Ltd. 1 March 2012 [24 January 2014]. (原始內容存檔於24 November 2020).
- ^ 5.0 5.1 Zytiga 500 mg film-coated tablets - Summary of Product Characteristics (SmPC). electronic medicines compendium (emc). Datapharm. 4 March 2019 [15 November 2019]. (原始內容存檔於15 November 2019).
- ^ Zytiga EPAR. European Medicines Agency (EMA). 13 March 2019 [15 November 2019]. (原始內容存檔於27 December 2020).
- ^ 7.0 7.1 7.2 7.3 Benoist GE, Hendriks RJ, Mulders PF, Gerritsen WR, Somford DM, Schalken JA, van Oort IM, Burger DM, van Erp NP. Pharmacokinetic Aspects of the Two Novel Oral Drugs Used for Metastatic Castration-Resistant Prostate Cancer: Abiraterone Acetate and Enzalutamide. Clin Pharmacokinet. November 2016, 55 (11): 1369–1380. PMC 5069300
 . PMID 27106175. doi:10.1007/s40262-016-0403-6.
. PMID 27106175. doi:10.1007/s40262-016-0403-6.
- ^ 8.0 8.1 8.2 8.3 Meeting Library - Meeting Library. meetinglibrary.asco.org. [9 September 2016]. (原始內容存檔於20 September 2016).
- ^ Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. Journal of Medicinal Chemistry. June 1995, 38 (13): 2463–2471. PMID 7608911. doi:10.1021/jm00013a022.
- ^ 10.0 10.1 10.2 10.3 Abiraterone Acetate Monograph for Professionals. Drugs.com. [15 November 2019]. (原始內容存檔於6 May 2012) (英語).
- ^ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 Zytiga- abiraterone acetate tablet, film coated. DailyMed. 13 June 2019 [15 November 2019]. (原始內容存檔於13 November 2014).
- ^ Scowcroft H. Where did abiraterone come from?. Cancer Research UK. 2011-09-21 [2011-09-28]. (原始內容存檔於25 September 2011).
- ^ World Health Organization. World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Abiraterone. Drugs.com. [14 April 2018]. (原始內容存檔於30 November 2014).
