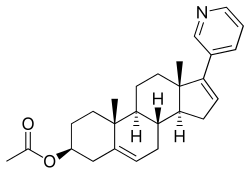
醋酸阿比特龙
外观
 | |
 | |
| 临床资料 | |
|---|---|
| 读音 | a" bir a' ter one |
| 商品名 | Zytiga, Yonsa, others |
| 其他名称 | CB-7630; JNJ-212082; 17-(3-Pyridinyl)androsta-5,16-dien-3β-ol acetate, abiraterone (BAN UK), abiraterone acetate (JAN JP), abiraterone acetate (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611046 |
| 核准状况 | |
| 怀孕分级 | |
| 给药途径 | 口服给药[2][3] |
| 药物类别 | 化学疗法 |
| ATC码 | |
| 法律规范状态 | |
| 法律规范 |
|
| 药物动力学数据 | |
| 生物利用度 | 不明,但空腹时可能最多50%[7] |
| 血浆蛋白结合率 | 阿比特龙:~99.8%(与白蛋白和α1 酸性糖蛋白)[7][2][8] |
| 药物代谢 | 酯酶、CYP3A4、SULT2A1[8] |
| 代谢产物 | 阿比特龙、其它[2][7] |
| 生物半衰期 | Abiraterone: 12–24小时[2][7][3] |
| 排泄途径 | 粪便:88%[2][8] 尿:5%[2][8][3] |
| 识别信息 | |
| |
| CAS号 | 154229-18-2 (154229-19-3(阿比特龙)) |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.063 |
| 化学信息 | |
| 化学式 | C26H33NO2 |
| 摩尔质量 | 391.56 g·mol−1 |
| 3D模型(JSmol) | |
| 熔点 | 144至145 °C(291至293 °F) [9] |
| |
| |
醋酸阿比特龙(英语:abiraterone acetate)是治疗前列腺癌的口服药物[10]。会与皮质类固醇并用于治疗转移性去势疗法无效型的前列腺癌(metastatic castration-resistant prostate cancer,mCRPC)和转移性去势疗法敏感型的高风险前列腺癌(metastatic castration-sensitive prostate cancer,mCSPC)[11]。 应使用于睾丸切除(去势疗法)后,或需与促性腺素释素调节剂并用[11]。
常见副作用包括疲劳、呕吐、头痛、关节痛、高血压、水肿、低钾血症、高血糖、潮热、腹泻和咳嗽[10] [11]。其他严重副作用包括可能肝衰竭和肾上腺皮质功能不全[11]。对伴侣可能怀孕的男性,应采取避孕措施[11]。醋酸阿比特龙服用后,在体内转化为阿比特龙[11]。它是一种雄性激素抑制剂,具体来说是CYP17A1抑制剂之一,作用后会减少睾酮的合成[10]。因此,可以避免这些激素刺激前列腺癌细胞的增生[10]。
醋酸阿比特龙的文献在 1995 年出现,于 2011 年在美国和欧洲取得医疗使用许可[12] [11]。名列世界卫生组织基本药物标准清单[13]。此药在世界各地都有销售[14]。
参考文献
[编辑]- ^ Abiraterone Use During Pregnancy. Drugs.com. 13 March 2020 [8 June 2020]. (原始内容存档于25 November 2020).
- ^ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Zytiga- abiraterone acetate tablet, film coated. DailyMed. 13 June 2019 [15 November 2019]. (原始内容存档于13 November 2014).
- ^ 3.0 3.1 3.2 3.3 Yonsa- abiraterone acetate tablet. DailyMed. 5 June 2018 [15 November 2019]. (原始内容存档于13 August 2020).
- ^ 4.0 4.1 4.2 Zytiga abiraterone acetate product information (PDF). TGA eBusiness Services. Janssen-Cilag Pty Ltd. 1 March 2012 [24 January 2014]. (原始内容存档于24 November 2020).
- ^ 5.0 5.1 Zytiga 500 mg film-coated tablets - Summary of Product Characteristics (SmPC). electronic medicines compendium (emc). Datapharm. 4 March 2019 [15 November 2019]. (原始内容存档于15 November 2019).
- ^ Zytiga EPAR. European Medicines Agency (EMA). 13 March 2019 [15 November 2019]. (原始内容存档于27 December 2020).
- ^ 7.0 7.1 7.2 7.3 Benoist GE, Hendriks RJ, Mulders PF, Gerritsen WR, Somford DM, Schalken JA, van Oort IM, Burger DM, van Erp NP. Pharmacokinetic Aspects of the Two Novel Oral Drugs Used for Metastatic Castration-Resistant Prostate Cancer: Abiraterone Acetate and Enzalutamide. Clin Pharmacokinet. November 2016, 55 (11): 1369–1380. PMC 5069300
 . PMID 27106175. doi:10.1007/s40262-016-0403-6.
. PMID 27106175. doi:10.1007/s40262-016-0403-6.
- ^ 8.0 8.1 8.2 8.3 Meeting Library - Meeting Library. meetinglibrary.asco.org. [9 September 2016]. (原始内容存档于20 September 2016).
- ^ Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. Journal of Medicinal Chemistry. June 1995, 38 (13): 2463–2471. PMID 7608911. doi:10.1021/jm00013a022.
- ^ 10.0 10.1 10.2 10.3 Abiraterone Acetate Monograph for Professionals. Drugs.com. [15 November 2019]. (原始内容存档于6 May 2012) (英语).
- ^ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 Zytiga- abiraterone acetate tablet, film coated. DailyMed. 13 June 2019 [15 November 2019]. (原始内容存档于13 November 2014).
- ^ Scowcroft H. Where did abiraterone come from?. Cancer Research UK. 2011-09-21 [2011-09-28]. (原始内容存档于25 September 2011).
- ^ World Health Organization. World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Abiraterone. Drugs.com. [14 April 2018]. (原始内容存档于30 November 2014).
